PROCESS & QUALITY MANAGEMENT
Successfully implementing process and quality management
The cross-industry DHC VISION solution for holistic process and quality management combines user-friendly process modelling and modern process management concepts with a powerful management of documents and risks as well as highly performant business intelligence and data analytics functions. DHC VISION stands for “Efficient and Compliant Quality”.
As process and quality management solution, DHC VISION PQM provides user-friendly tools and methods for modelling, communicating, measuring, and continuously improving the entire process landscape. The process pyramid provides a variety of predefined modelling notations on all levels; common notations and objects are also available for mapping and integrating the organizational structure. The direct integration of the standard documentation is achieved by means of comprehensive DMS functionalities. Workflows and electronic signatures ensure the secure control and release of documents and processes. With regard to compliance with ISO 9001 requirements, risks, associated controls and measures can be documented in a process-oriented manner.
Measures and improvement potentials actively involve employees; they also support continuous improvement. DHC VISION is an Integrated Management System (IMS), covering a variety of different standards and norms. Interactive analysis scenarios allow continuous monitoring of quality assurance measures and the control of the most important industry quality metrics (KPIs). Information relevant for decision-making is visualized in role-specific dashboards.
Request factsheet
Compact information on all processes related to controlled documents and the complete range of functions is available in the factsheet on DHC VISION SOP Management.
"*" indicates required fields
Impressive functionality
Process landscape
- Design of a consistent process landscape
- Hierarchical mapping of organizational structure and process organization
- Comprehensive selection of modelling methods
- Modelling via drag & drop with preset shapes
- Database- and method-supported modelling
- Re-use of objects (jobs, roles, IT systems, etc.)
- Automatic generation of organizational and process instructions
- Real-time publication of model graphics
- Graphical navigation over organization and process models
- Seamless integration of documents, risks, and improvement suggestions
Document management
- Comprehensive support for document management
- Provision of all common document types
- Document templates with customer-specific design
- Upload and editing of any document format (MS Office, PDF, etc.)
- MS Office integration
- Best-practice workflows for, e.g., editing, review, and approval processes
- Notification function for new and changed documents
- Clear dashboards for monitoring status and validity period
Risk / improvement management
- Documentation and detailed presentation of operational and strategic risks
- Quantitative and qualitative risk assessment
- Risk strategy definition
- Automatic creation of risk heat maps including gross and net comparison
- Workflow-supported processing of measures for minimizing risks
- Description of proposals for improvement and correction
- Evaluation of suggestions and expected potentials for optimization
- Notification of the document or process owner of incoming suggestions
- Planning and definition of implementation deadlines
Insight into our costumer relations

“Through DHC VISION, we can roll out our process management worldwide in a standardized way and still have the flexibility to take local specifics into account without much effort.”
Thomas Schürmann
Beumer Group
All options at a glance
Process landscape
- Design of a continuous process landscape with integration of all relevant company information
- Hierarchical process structure from process step to enterprise process
- Provision of a variety of modelling methods for mapping processes and organizational structure: Process map, process diagram, process flow diagram / value chain diagram, supplier-input-process-output-customer (SIPOC), business process diagram, swim lane, BPMN 2.0, event-driven process chain (EPC), organizational chart, target diagram
- Level-specific definition of process methods
- Modeling via drag & drop
- Preset modelling shapes
- Use of MS VISIO for process modelling
- Database- and method-supported modelling and reuse of objects
- Definition of responsibilities
- Predefined and customizable attribute definitions
- Attribute-specific versioning and version management
- Publication of new processes
- RACI methodology
- Process cockpits for role-specific representation of required information
- Automatic creation of hierarchical process and organizational structures
- Easy navigation and drill-down function in the models
- Seamless integration of documents, risks, controls, and improvement suggestions
- Tabular, visual comparison of models
Document management
- Comprehensive document management system for process-oriented management and control of standard documents (cf. DHC VISION SOP Management)
- Provision of common document types (work instructions, procedural instructions, guidelines, etc.)
- Document templates with customer-specific design
- Upload and editing of any document formats (MS Office, PDF, image and video formats)
- MS Office integration
- Audit-proof storage of documents in freely definable structures
- Import of controlled documents
- Best-practice workflows for, e.g., editing, review, and release processes
- Predefined (release) procedures for invalidation, validity extension, reactivation and archiving of documents
- Real-time publication
- Full document reference and usage tracking to support document invalidation by authorized users (groups)
- Automatic triggering of revision processes
- Dynamic display and easy editing of document-related correction suggestions
- Simple definition of responsibilities and areas of validity
- System-supported monitoring of document validity
- Versioning and historization of all published document versions
- Automatic creation of a change history
- Comment function and document for communication between initiator, creator, reviewer, and releaser
- Clear management and control of all document-related suggestions for correction
- Automatic conversion of documents to PDF
- Target group-oriented information distribution for new or changed documents (for information / for attention)
- Option to integrate the DHC VISION training management for closed document life cycle processes
Risks, opportunities and controls
- Documentation and detailed presentation of operational and strategic risks, incl. predefined description set
- Use of reference catalogues for risks, measures and controls
- Quantitative and qualitative assessment (amount of damage, probability of occurrence, expected value of damage); gross and net analysis (before and after measures)
- Risk strategy definition
- Risk maps for processes, organizational units, and IT applications
- Determination of risk dependencies and creation of a risk pyramid including aggregation and consolidation of risks
- Generation of risk portfolios (e.g., per process)
- Automatic generation of risk heat maps incl. gross and net comparison
- Control of risks by defining and assigning suitable measures, controls or issuing guidelines
- Workflow-supported processing of optimization or implementation measures including reminder and escalation mechanisms
- Reactive triggering of workflow upon risk occurrence
CIP
- Optional integration of DHC VISION CAPA Management
- Suggestions for improving and correcting processes and documents
- Evaluation of suggestions and expected optimization potentials
- Notification of the document or process owner of incoming suggestion
- Graphical representation for the author if suggestions are still available and unprocessed
- Planning and definition of implementation deadlines
- Automatic status management
- Initiation of optimization or implementation measures and control via workflows
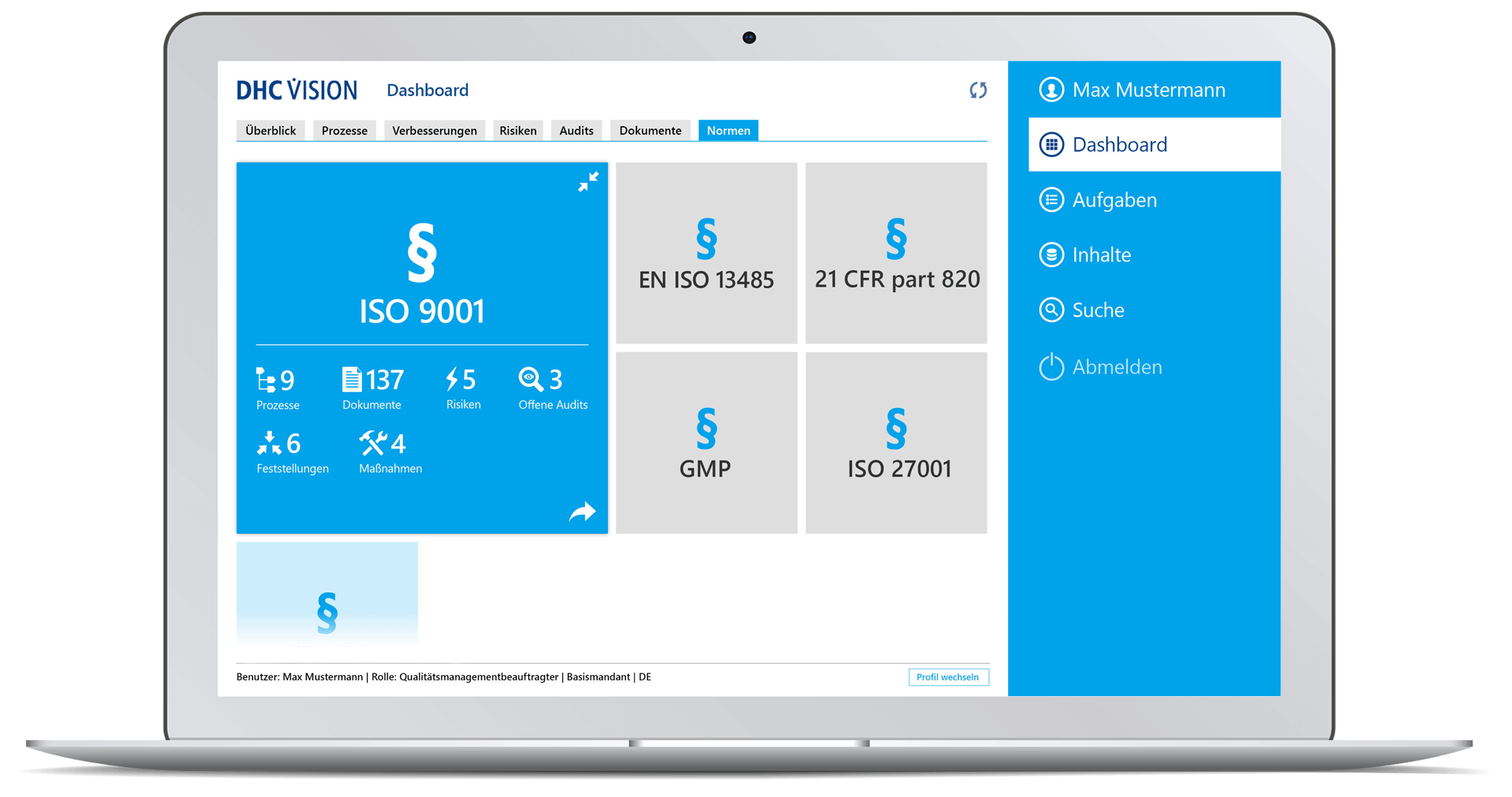
Norms and standards
- Simple mapping of norms, standards or laws, their chapter structure and, if applicable, content
- Subdivision of regulations into chapters and subchapters
- Intuitive navigation through standards
- Use of chapters as master data and linking norm chapters to processes, documents, audits, risks, measures, and findings
- Multi-perspective display of all objects related to the standard
- Functions for convenient updates of standards
- Life-cycle management and evaluation of compliance with and the up-to-dateness of the standards, incl. monitoring of the complete mapping of all processes and documents
Multisite and Multilinguality
- Intelligent client concept for the international context
- Methodology for harmonizing and standardizing processes and procedures with options to adapt them to local needs
- Intuitive variant management (global and site-specific variants)
- DE and EN as standard languages and language packages for international use (unicode-enabled)
- Special profiles for easy translation of processes, documents, or other content
- Provision of a translation workflow for formal approval of language variants
- Easy switching between languages
Events, notifications, communication
- Notification Event Modeling Framework for automated, accurate and timely notification of people, roles/groups or systems about the status value of definable events such as date, threshold, metric, new document versions.
- Flexible and appealing design of notifications (including HTML); also multilingual, to different recipient systems (email, social media, mobile gadgets etc.
- Rules and communication by creating messages along role-based interests and views (user view, organizational view, compliance view).
- All notifications are subject to an audit trail
- Full traceability of who was informed about what, when, with what content
Monitoring and search
- Standard reports on the status of processes, organizational charts, documents, notices, change proposals, (paper) copies or for planning resubmissions and revisions
- RACI matrix for analysis and representation of responsibilities (of one or more processes)
- Process model comparison and variant comparison
- Reports for staffing and activity-based costing
- Detailed process reporting
- Export to MS Excel of selected reports
- Risk maps for processes, organizational units, and IT applications
- Automatic generation of risk portfolios and gross/net risk heat maps
- High-performance cognitive search with context-sensitive prioritization of search results
- Comprehensive full-text search with a wide range of filter and selection functions
Analytics and Business Intelligence
Role-specific dashboards with standard apps and relevant information
Processes
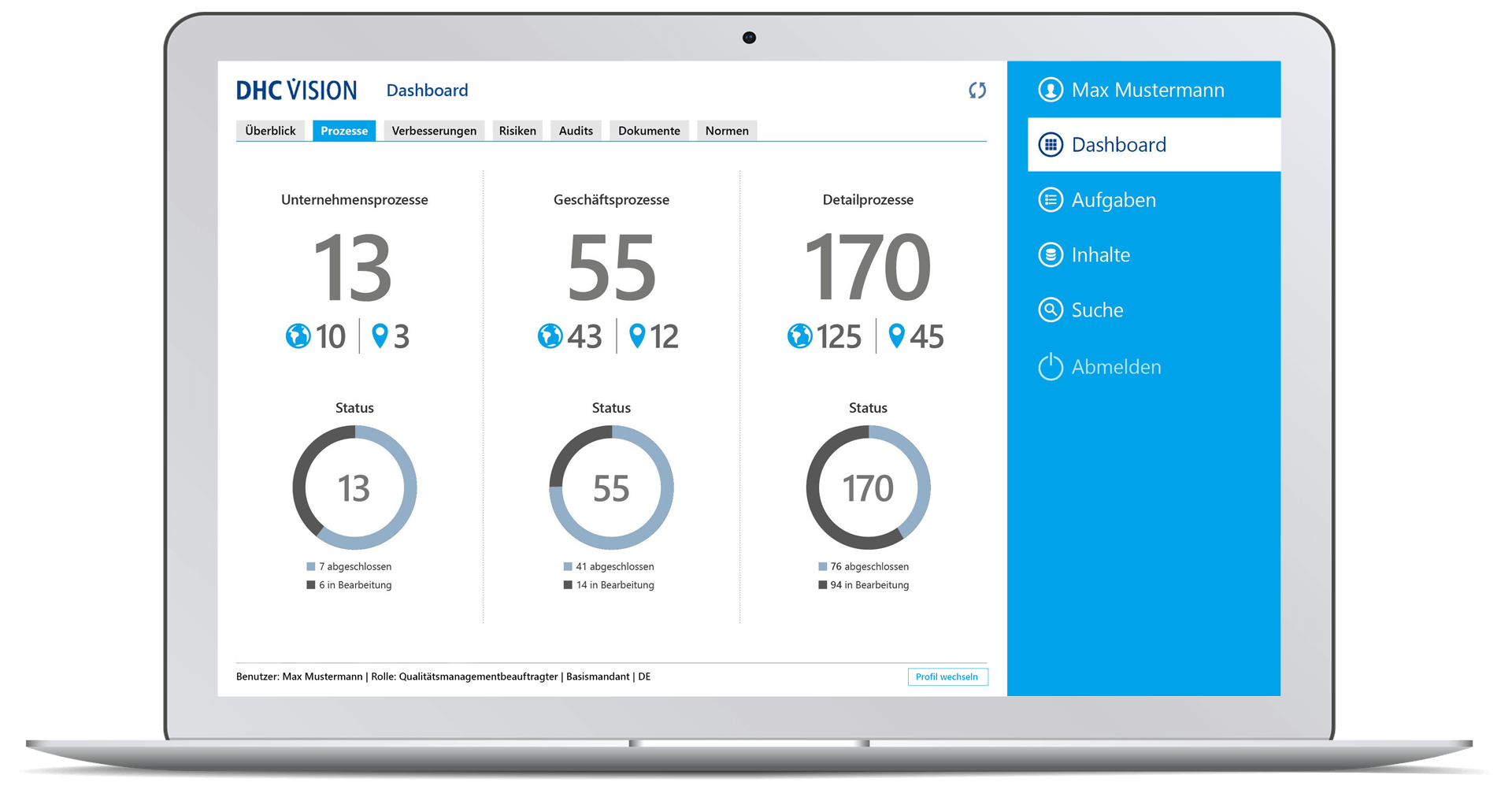
- Number of company processes, business processes and detailed processes incl. visualization of the localization (clients) and display of their status.
- Drilldown to a single process with status information on version, process type, valid from/to and details on the number of upstream processes, existing process models, documents, or subsequent processes (each including navigation option)
- Quickview of the process
- Access to the process cockpit with all process-relevant information
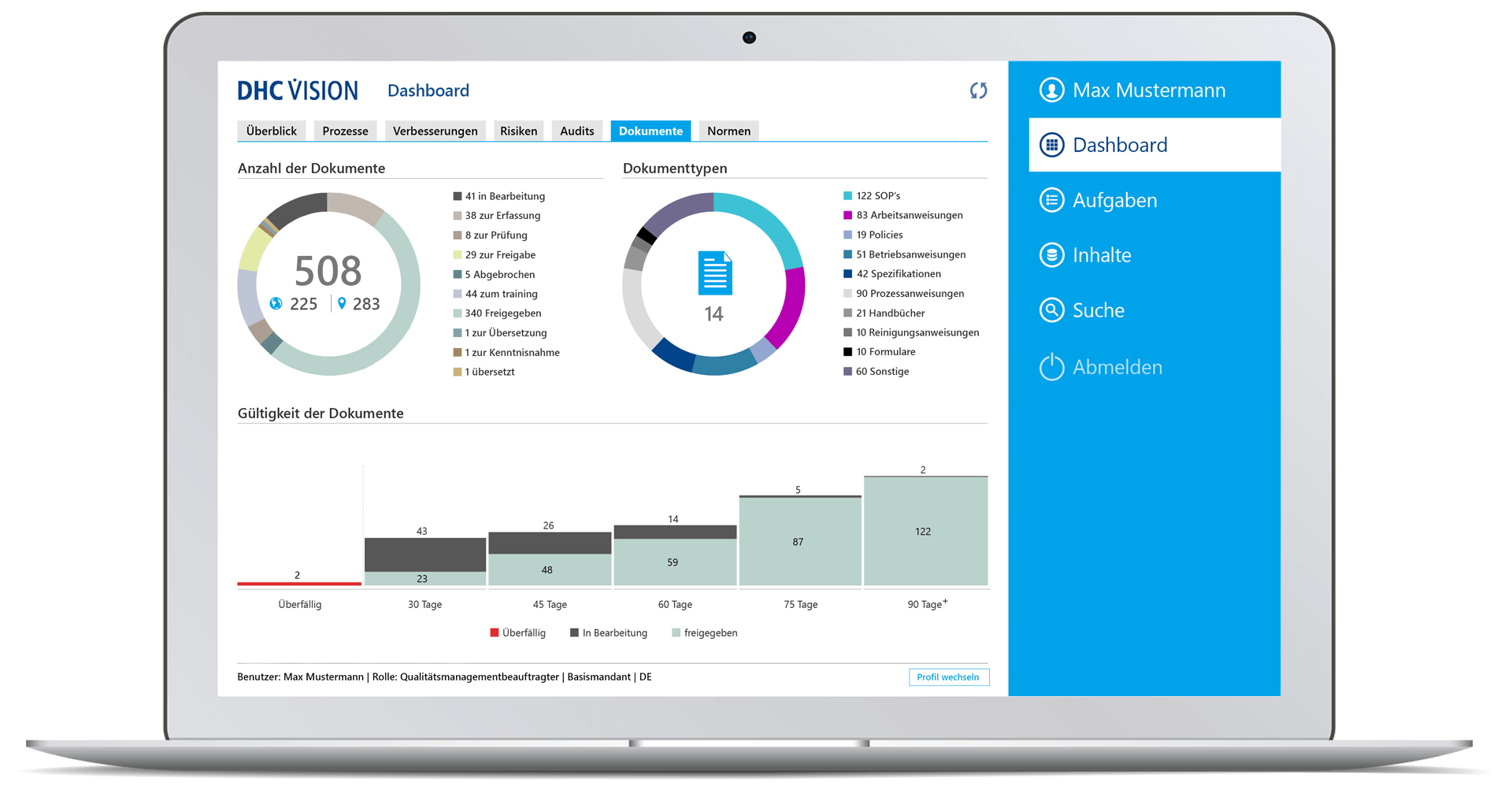
Documents
- Number of documents and their status, breakdown by document type and number of documents per document type.
- Overdue management: Validity of documents incl. 90 days forecast display
- Drilldown to individual document level with status information on version, life cycle status, document type, last change, “my role” and details such as number of references, attachments, variants, or language versions
- Quick view of documents and applicable attachments
- Direct access to documents with a view of core information such as applicable attachments, proposed changes, version history, distribution list, status for acknowledgement (persons, deadline, target date, completion date), references, comment documents, where-used list, change history, access to older versions
Improvements and standards
- Number of processes, number of improvement potentials, number of measures
- Presentation of the status of processes (in progress, completed)
- Overview of relevant norms, standards, and laws
- Presentation of standard-related content with assigned processes and documents, upcoming/ongoing audits as well as documented findings and ongoing measures
Risks
- Visualization of the average expected loss value as well as the total sum of all expected loss values
- Number of released risks and in their processing status
- Graphical representation of the number of initiated measures to reduce risks and their processing status
- Drilldown with list-like view of risks with their value, type and assigned measures
Compliance and security
- Audit-proof storage of all information objects and data
- Comprehensive authorization concept to ensure the highest security standards
- Timely notification/reminder to owners of documents or processes before expiration of validity periods
- Escalation triggered when critical points in time are reached (definable per object type)
- Encryption – documents that have already been signed or released can no longer be edited in the respective version
- Deactivation and reactivation of user accounts
- Controlled, traceable workflows for company-wide systematization
- Mobile-capable
- Comprehensive master data management
- For Life Science:
- Compliance with 21 CFR Part 11 (Electronic Records, Electronic Signatures)
- Validation Package (services and content for GxP/ FDA compliance)
Validation and compliance consistently in view
DHC VISION is specially designed for use in highly regulated industries. The solution meets GxP guidelines and directives of the FDA, EMA, PIC/S or ICH, as well as 21 CFR Part 11, for both technology and business processes. The “Validation Package” consists of “Validation Accelerators” (complete documentation set for validation) and Validation Services for adapting the documentation to a specific system configuration.
Matching products
SOP CONTROL
The optimal solution for digital management and control of your specification documentation. Secure, controlled, traceable and compliant (including 21 CFR Part 11).
TRAINING
The perfect and seamlessly integrable addition to SOP management. Digital processes set new standards in “Training Compliance”.
Your information package
Get an impression of this and other products or read what insights we have gained from research and development. Take advantage of our exclusive content such as white papers or study results on the digitization of quality and compliance processes. Put together your desired media easily and conveniently.
Worth knowing | News | Latest
DREWAG and ENSO launch document management with DHC Business Solutions
The East Saxon energy suppliers DREWAG - Stadtwerke Dresden GmbH and ENSO Energie Sachsen Ost AG have been using the electronic document...
Digital Transformation in Quality Management at Allessa with DHC VISION
Allessa GmbH is one of the leading contract manufacturers of organic intermediates and specialty chemicals. Allessa now relies on the...
Endress+Hauser to deploy DHC VISION process and quality management solution company-wide in the future
More efficient management of business processes, documents and audits To standardize its process management activities, Endress+Hauser, a...
FAQ
What are the methods for process modeling?
There are different modeling methods for mapping the process and organizational structure. These include: Process Map (PLK), Process Diagram, Process Flow Diagram / Value Chain Diagram, Supplier-Input-Process-Output-Customer (SIPOC), Business Process Diagram, Swimlane, BPMN 2.0, Event-driven Process Chain (EPC), Organizational Chart, Target Diagram. The methods capture and document organizational structures and internal company processes. In a process model, the process map is the top level; at further levels, processes are represented in increasing detail.
How are process and quality management related?
For an effective quality management, it is essential to document processes, to continuously monitor them, and to implement improvements to the extent needed. Only when it is clear how processes are designed can irregularities in process flow and process execution be detected; deviations from the defined target state, which is to lead to the desired quality of results, can be identified. Accordingly, it is important that the specification documentation – instructions, manuals, forms, etc. – is integrated into processes and that risks, controls and measures are documented in a process-oriented and traceable manner. The latter is required by the ISO 9001 standard and its industry-specific variants.
What is meant by "quality oversight"?
“Quality oversight” refers to the continuous and systematic observation of all GxP-relevant processes in a company by the corporate unit responsible for quality management. The corresponding activities are related to the overriding concern of ensuring the quality and safety of medical devices. Effective “Quality Oversight” relies on detailed and comprehensive documentation of relevant processes; it requires access to data from process execution. On this basis, critical developments and incidents can be identified and analyzed; appropriate measures can be initiated to eliminate deficiencies or to minimize risks.