REGULATORY COMPLIANCE
Next generation modular software solutions for compliance
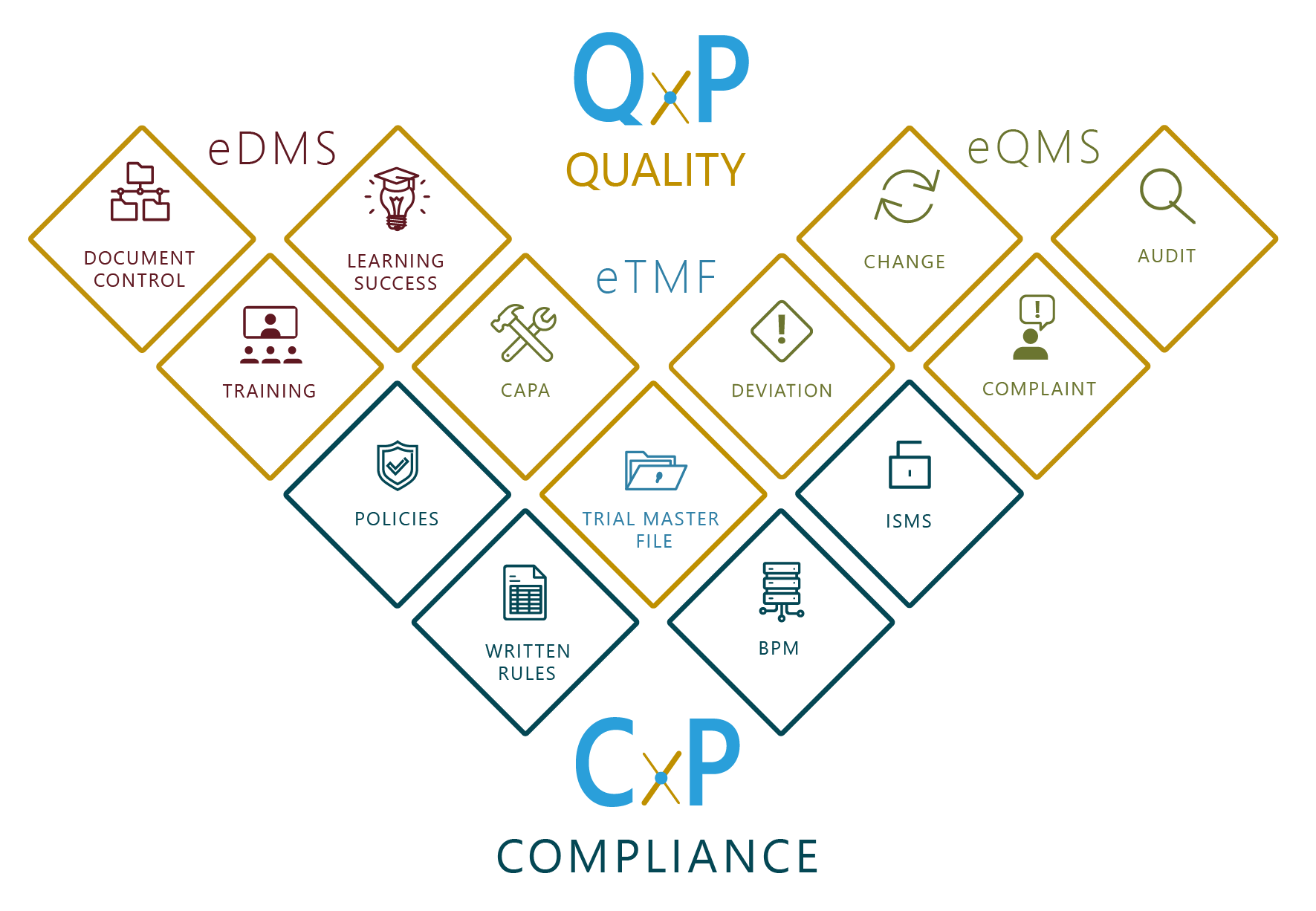
CxP integrates compliance instructions and standards as well as normative and legal processes in a modular platform. This allows written rules of procedure, guidelines or policies to be designed in accordance with regulatory requirements. The digital implementation of quality standards (9001, 13485, etc.), information security (2700x) and data protection (according to EU-DSGVO ) rules fosters the change of perspective from an internal compliance and security architecture to the normative world.
Laws and comprehensive regulations aim to avert risks and damage to companies; they ensure the effectiveness, efficiency, and regularity of business activities and operations. All software modules follow the PDCA cycle with automated control and monitoring processes and workflows. Role-specific dashboards, business intelligence and data analytics tools as well as comprehensive reporting features provide solid information about the company’s compliance readiness. With “AI inside” (artificial intelligence such as machine learning or blockchain), time-consuming routine activities become obsolete.
Validation and compliance without compromise
DHC VISION is specially designed for use in highly regulated industries. The solution fulfills GxP guidelines; technical implementation and business processes are in line with FDA, EMA, PIC/S or ICH directives as well as with 21 CFR Part 11. A solution package is available for system validation. It includes comprehensive documentation for rapid software validation (“Validation Accelerators”); professional validation services respond to specific and complex validation requirements.
Discover the CxP | Compliance products
POLICIES
Designed for digital creation, maintenance and communication of company policies and procedures. Secure, controlled and traceable at all times.
PROCESSES
More than just modelling. The solution combines modern process management concepts with a powerful DMS and a more than standard-compliant risk management.
Insight into our costumer relations

“We are now focusing on digitization and, thus, can handle the more complex GxP requirements in a cost-neutral way. Our growth is triggered by value-creating processes rather than G&A costs. We have significantly reduced turnaround times for GxP-relevant documents, need less resources for manual process handling, see more process efficiency and significantly lower risks.”
Thomas Dürre
ITM Isotopen Technologien München AG
Your information package
Get an impression of this and other products or read what insights we have gained from research and development. Take advantage of our exclusive content such as white papers or study results on the digitization of quality and compliance processes. Put together your desired media easily and conveniently.
News
RegTech insights: Digitization of Post-Market Surveillance: Ramp-up for the AI service platform SmartVigilance.
Saarbrucken, January 2023: After two years of research and development work, the ramp-up phase for the data platform SmartVigilance is now...
RegTech insights: Validate software with artificial intelligence
Artificial intelligence makes computer software validation (CSV) easier, more reliable, less expensive and more efficient. This is the...
Discussion of the DHC innovation strategy with Minister President Tobias Hans
Today, the development and production of pharmaceutical and medical technology products are no longer conceivable without digital...